Description
Storage of the Kit.
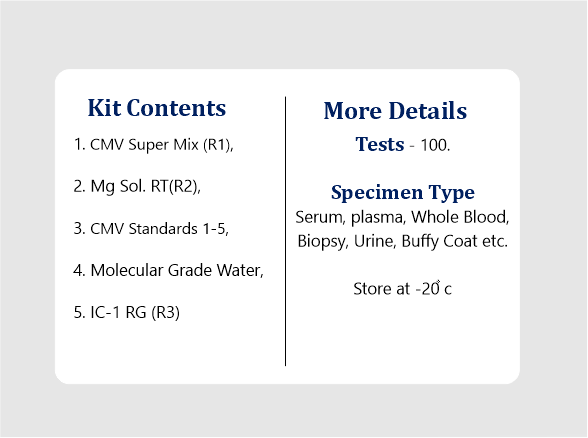
All the kit’s reagents should be stored at -20°C and stable till expiry at this temperature. Repeated thawing and freezing (> 3x) should be avoided, as this may reduce the assay’s sensitivity. If the kit is to be used only occasionally, the reagents should be frozen in aliquots. Storage at +4°C is not recommended & should not exceed a period of 2 hours in any case.
GENO SEN’S CMV RNA QUANTITATIVE PCR KIT
PRODUCT’S BENEFITS:- WHAT’S SPECIAL ABOUT US:-
HIGH SPECIFICITY
- The kit detects all known CMV.
- 100% diagnostic specificity & sensitivity.
- Dual targeting prevents detection failures caused by the occurrence of mutations.
UNIQUE FALSE-NEGATIVE RESULTS CONTROL
- Unique Internal DNA control construction.
- Controls the whole diagnostic process, including DNA extraction, reverse transcription, and PCR amplification.
EASY-TO-USE
- Single tube Ready-to-Use Master Mix contains all components for PCR amplification with 5 levels of standards.
- No additional PCR reagents pipetting is necessary.
HIGH QUALITY
- All diagnostic kits manufactured by GENOME DIAGNOSTICS PVT LTD are under strict quality control guidelines.
SPECIFICATION:-
| Specimen Type | Serum, Plasma, Whole Blood, Biopsy, Urine, Buffy Coat, |
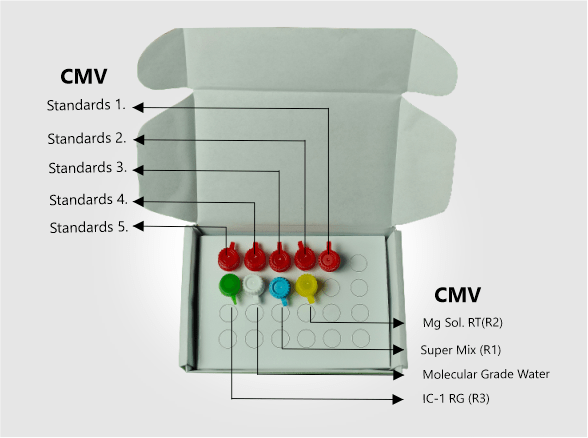
| Kit Contents | CMV Super Mix (R1), Mg Sol. RT(R2), CMV Standards 1-5, Molecular Grade Water, IC-1 RG (R3) |
| Instrumentation | Real-Time PCR Kits are compatible with all open systems i.e. Biorad, Qiazen, ABI, Roche, Rotor Gene™ 2000/3000/6000, etc. |
| Tests | 100 Reactions. |
| Extraction Method | Spin Silica Membrane Column Based Method. |









Reviews
There are no reviews yet.